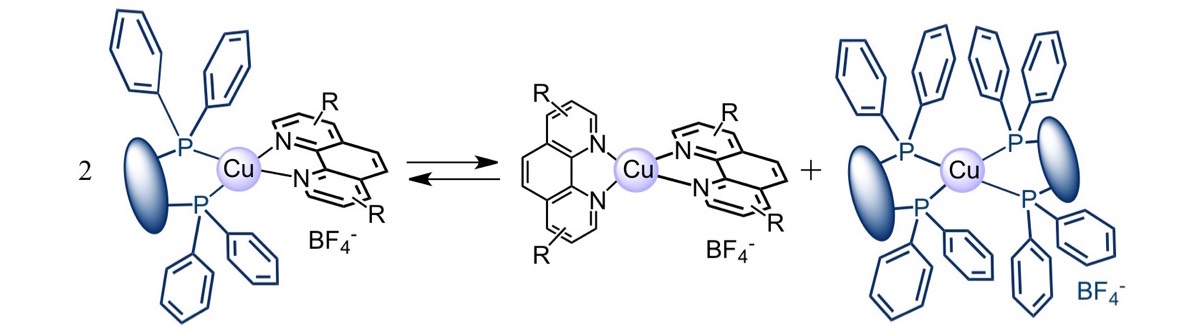
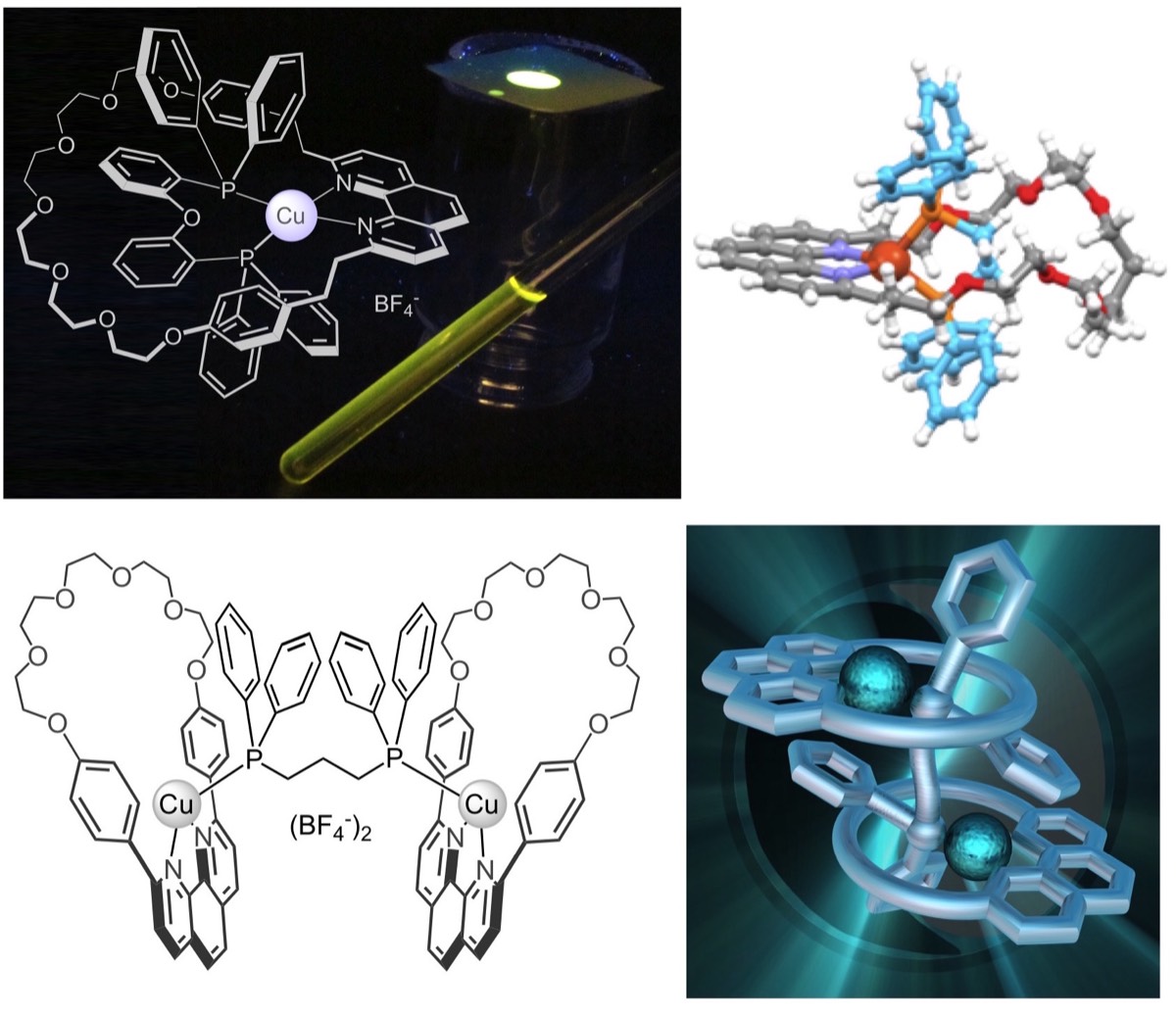
Coordination compounds possessing low-lying metal-to-ligand charge transfer (MLCT) excited states with marked reducing character are also excellent partners for [60]fullerene in photoactive multicomponent hybrid systems. We have prepared a large number of dyads in which [60]fullerene is coupled with photoactive coordination compounds of Ru(II), Re(I), Ir(III) and Cu(I). For most of these systems, the energy of the lowest triplet MLCT level lies higher than that of the fullerene singlet and triplet, whereas the charge separated state is intermediate. Upon excitation of the metal-complexed moiety, charge separation followed by charge recombination to the fullerene triplet is generally observed. Practically, since direct excitation of the fullerene moiety results in regular deactivation without intercomponent interactions, the fullerene triplet level is the final energy sink of the dyad, whatever the excitation wavelength. The situation is rather different in the case of Cu(I)-bisphenanthroline fullerene hybrids. Cu(I) complexes are indeed stronger reducing agents than Ru(II), Re(I) or Ir(III) systems, thus the charge separated state is the lowest in the energy level diagram, originating a different pattern of photoinduced processes.